Biology
Neutrons and the study of macromolecular structures

Watch Dirk Wallacher and Matthew Barrett on the new humidity chamber that will shed light on processes behind Alzheimer’s disease!
The human genome is believed to code for between 40,000 and 1000,000 proteins. In the current, post-genomic era, information resulting from genetic sequencing is being harnessed to reveal how these proteins are involved in complex cellular processes. Each protein does a particular job and to really understand life´s processes we must find out how all of them perform their role. We know that the shape and structure (the arrangements of atoms) of proteins is related to their function, and we are starting to realise that dynamic changes within a molecular structure might also be involved.
This is particularly important to treat illness, for example, as most diseases result from a malfunction at the molecular level – a structural defect resulting in an inactive enzyme, a pathogen blocking a crucial cellular process.
In recent years, neutron scattering has played an increasing role in the study of biological structures.
Neutron scattering methods
In biology, the main objects of study are large molecules such as nucleic acids, proteins, lipids or complex sugars such as carbohydrates, with sizes ranging from 1 to 10 nanometres.
The wavelength of neutrons makes them ideal to study such large molecules, or even macromolecular structures such as cell membranes or viruses.
In particular, small angle neutron scattering (SANS), which involves scattering neutrons at very small angles can be used to study complexes of several molecules.
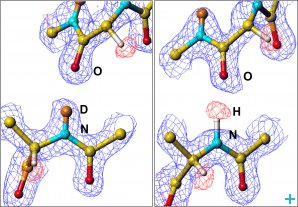
Another important characteristic of neutrons is their scattering power. Neutrons scatter off different atoms with different power, which allows us to see inside molecules.
A technique called contrast variation allows to highlight parts of a structure by replacing its hydrogen atoms with the stronger-scattering deuterium (a hydrogen isotope containing a neutron in its nucleus).
Another interesting characteristic of neutrons is that they can lose or gain energy to or from the atom they bounce against during scattering. This phenomenon, called inelastic scattering will set the atoms in motion, and can therefore reveal their motion patterns. This can be used to determine how a molecule moves and if it has flexible or rigid parts.
Related articles
- Biosensors will benefit from new method to produce membranes , NMI3 highlight
- NMI3´s Deuteration Joint Research Activity developed new methods to improve the scope and quality of biological neutron scattering experiments.
- NMI3's Advanced Neutron Tools for Soft and Bio-Materials Joint Research Activity is developing a platform for model biological membranes, soft-material-specific sample environments and in-situ devices.
- Proteins perform without water , NMI3 article
- Interview with Giovanna Fragneto, coordinator of the task A platform for model biological membranes for structural and dynamical characterisations of the Joint Research Activity (JRA) Advanced Neutron Tools for Soft and Bio-Materials.
- For examples of research in this field please visit the ILL or the ISIS biology pages.
Videos
A JRA supported by NMI3 is working on a new humidity chamber. The instrument will be used for neutron scattering experiments to reproduce the human body conditions in order to shed light on biological processes such as the development of Alzheimer’s disease.
During a neutron scattering experiment the sample might degrade and produce wrong results. In our new video, researchers explain their work on a set up to control the sample quality along time so that false data can be discarded. This work will improve the data quality.
Watch Dr. Trevor Forsyth talk about his work and the Deuteration Joint Research Activity he coordinates for NMI3, at the Institut Laue Langevin (ILL), in Grenoble.